PSMA update, access appear high for US-based urologists; Dr. Jason Hafron explains what this means for patients
Urologist access to prostate-specific membrane antigen (PSMA)-targeting agents is high among US practitioners, results of an exclusive Urology Times survey indicate.
In total, 88% of respondents said they can order PSMA scans locally, with 9% saying they could not, and 3% saying they were not sure (Figure 1).
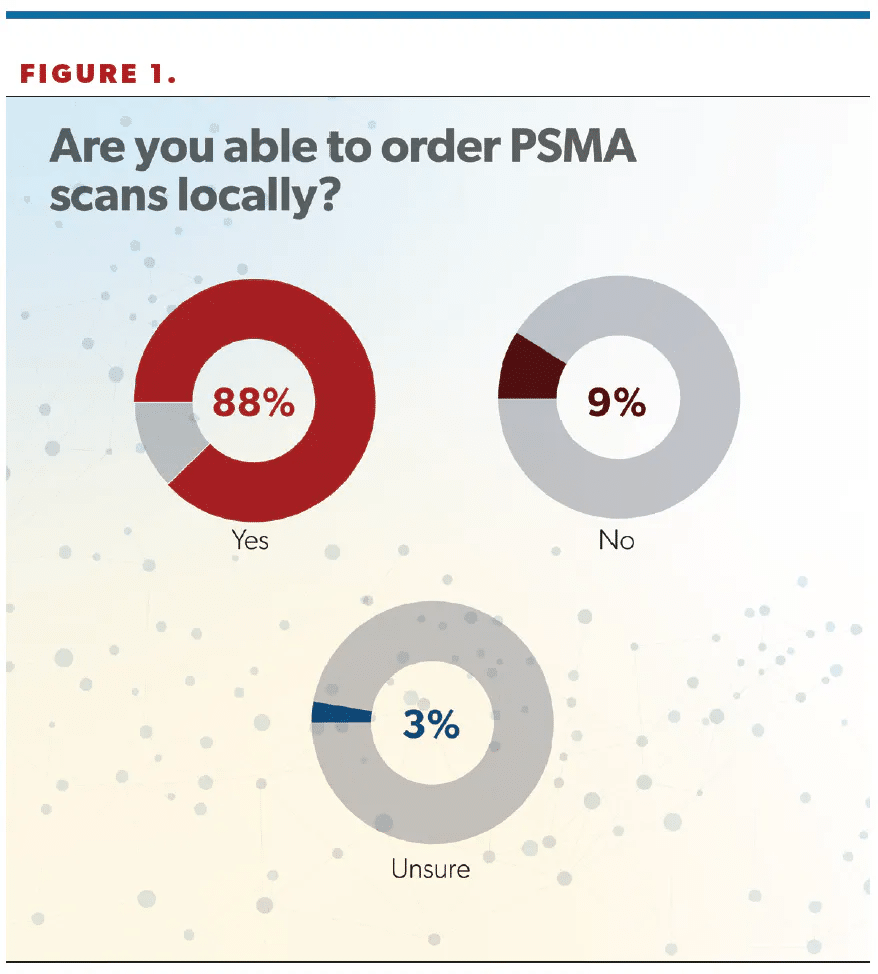
“I was glad to see that [88%] of US urologists were able to order a PSMA-PET [PSMA-positron emission tomography] study today. PSMA-PET is disruptive technology that allows doctors to see metastatic or recurrent prostate cancer earlier anywhere in the body. It’s great to see that urologists and, more importantly, our patients are getting access to these studies across the United States. This speaks to the extensive distribution networks of these products,” Jason M. Hafron, MD, told Urology Times. Hafron is the chief medical officer and medical director of clinical research at the Michigan Institute of Urology, PC, and a professor of urology at the William Beaumont School of Medicine, Oakland University, Rochester, Michigan.
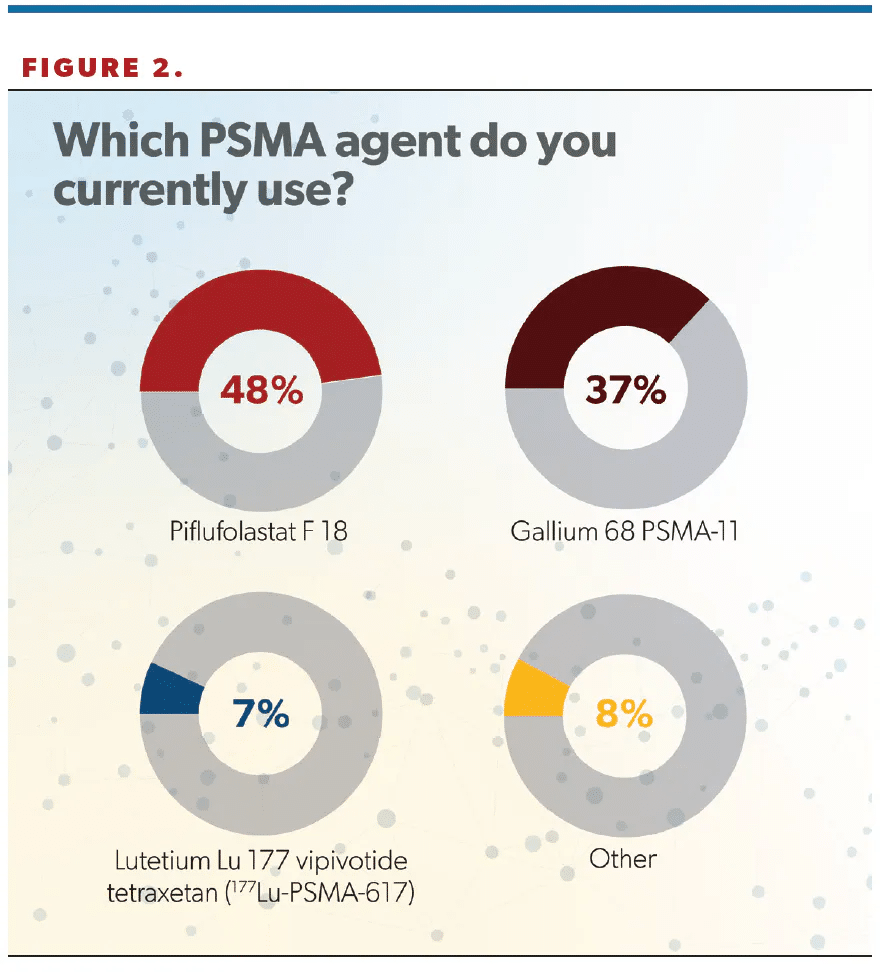
Nearly 50% of respondents said they used piflufolastat F 18 (Pylarify), whereas 37% selected gallium 68 PSMA-11 (68Ga-PSMA-11), 7% reported experience with lutetium Lu 177 vipivotide tetraxetan (177Lu-PSMA-617; Pluvicto), and 8% said “Other” (Figure 2). (Editor’s note: This survey was fielded prior to the May 30 FDA approval of flotufolastat F 18 [formerly 18F-rhPSMA-7.3; Posluma] for PET imaging of PSMA-positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum PSA [prostate-specific antigen] level.)
“It was also interesting to see that piflufolastat, or F 18, was the most common agent used in the United States. It was the first agent approved, in May 2021. F 18 does have a longer half-life and potentially may lead to more widespread availability as well as more convenient and flexible scheduling. Additionally, the radiophysical properties of F 18 allow for better imaging quality, and this may indicate why we’re seeing more F 18 used across the US,” Hafron said.
Urology Times Co–Editor in Chief Michael S. Cookson, MD, MMHC, FACS, who developed the survey, commented, “The gallium scans can be made more locally; there’s not as much logistics and also avoidance of that dependency on a cyclotron…. There’s probably some confusion about what some of the providers are ordering because when we looked at other things they were ordering, some of them just named the university they might be ordering from and things like that. So they may not actually know whether they’re using gallium or F 18.”
Cookson continued, “In some ways, maybe that doesn’t matter as much. There’s really not great head-to-head comparisons between the two. But a little bit of inferential data from the available stuff suggests maybe in that very low range of PSA detection, say, in the recurrent patients, maybe the F 18s are a little bit better quality for detection. But again, in general, [there are] not a lot of differences there.” Cookson is professor and chair of urology at the University of Oklahoma Health Sciences Center in Oklahoma City.
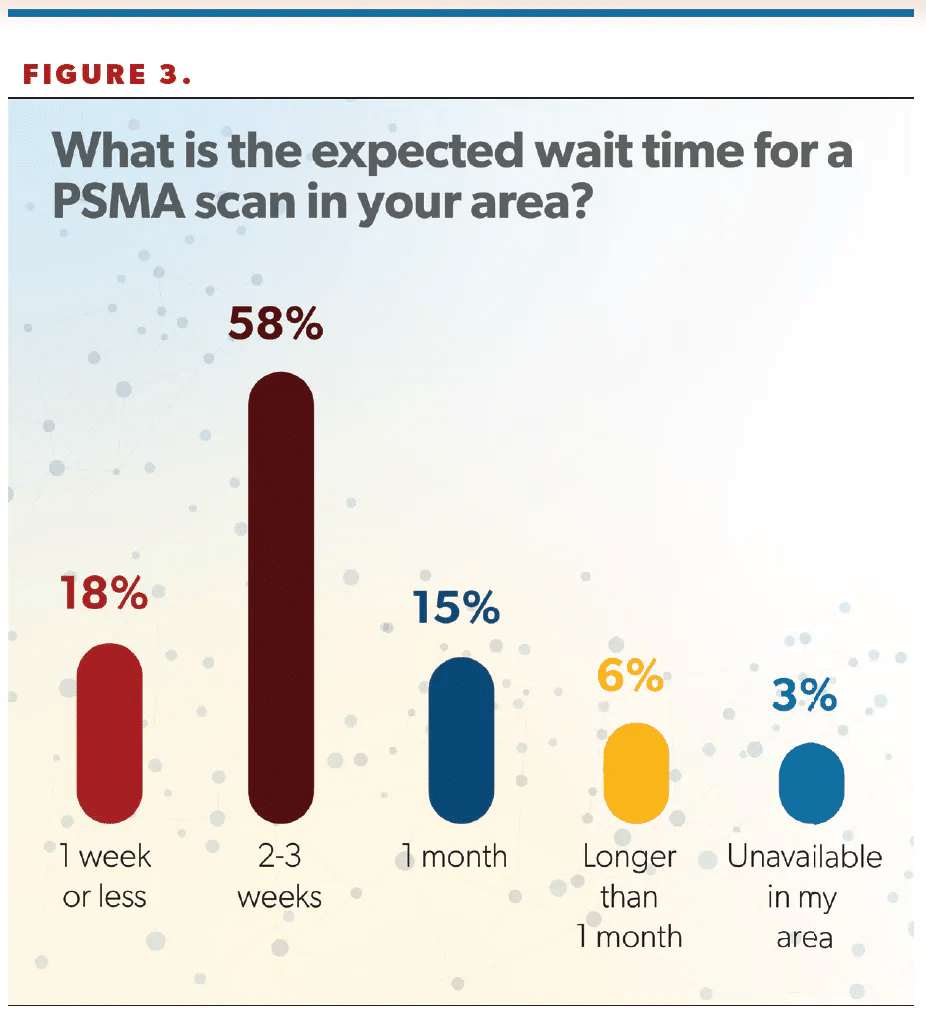
Regarding wait times for PSMA scans, more than half of respondents (58%) said they were able to obtain a scan for patients within 2 to 3 weeks (Figure 3). Hafron said this time frame aligns with his experience. “I think the frustrating part about this is it does require additional staff,” he said. “It’s the responsibility of the urologist, not the facility that you’re ordering that test from, to get that prior [authorization]. I know in our large LUGPA group, we’ve had to hire more staff to get these tests approved and to get prior [authorization]. So it is frustrating, it does delay things, but, ultimately, we are getting these tests approved.”
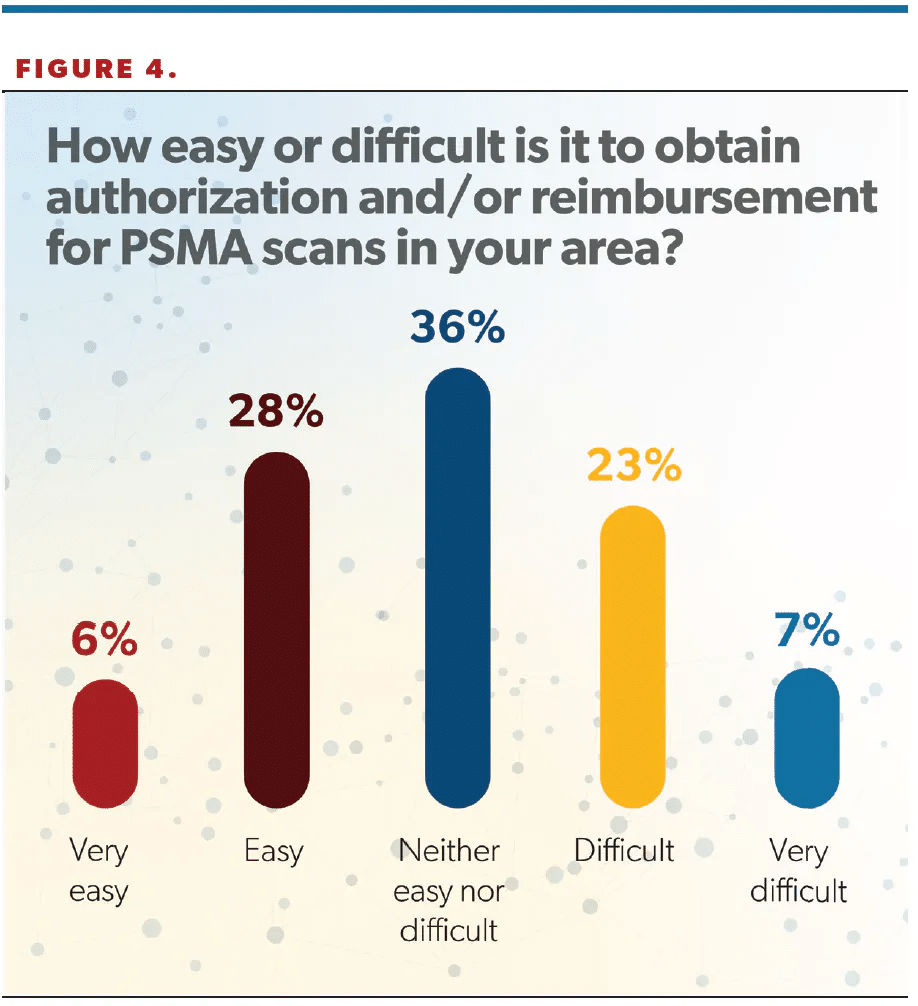
The survey included a question about prior authorization, with 36% of respondents saying it was “neither easy nor difficult” obtaining approval for scans, and 34% saying it was easy or very easy to obtain authorization (Figure 4).
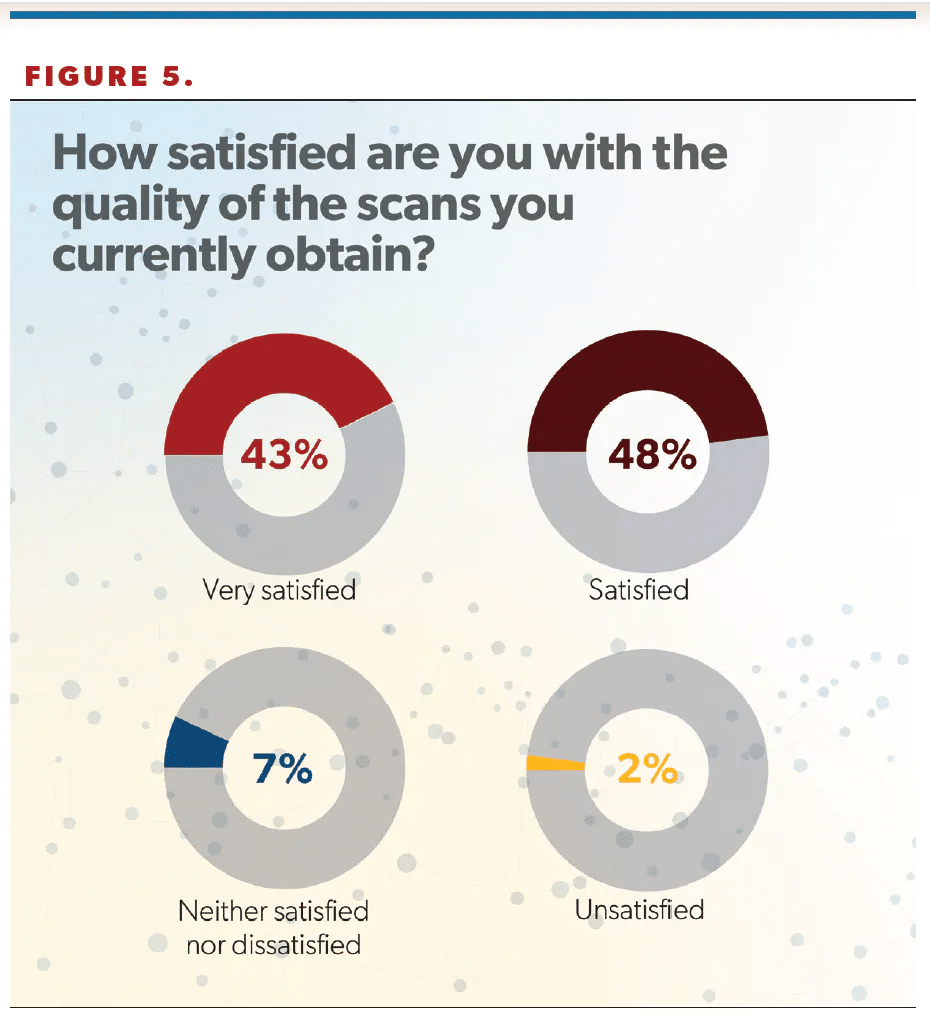
Provider satisfaction with PSMA scan quality was high, with 91% of respondents saying they were satisfied or very satisfied with the quality of scan they were able to obtain (Figure 5).
“That’s good news,” Cookson said of the high satisfaction rate. “You hate to order something where you’re really dissatisfied, or it’s very ambiguous. So that gives some good data to support that maybe they’re getting what they’re actually looking for there.”
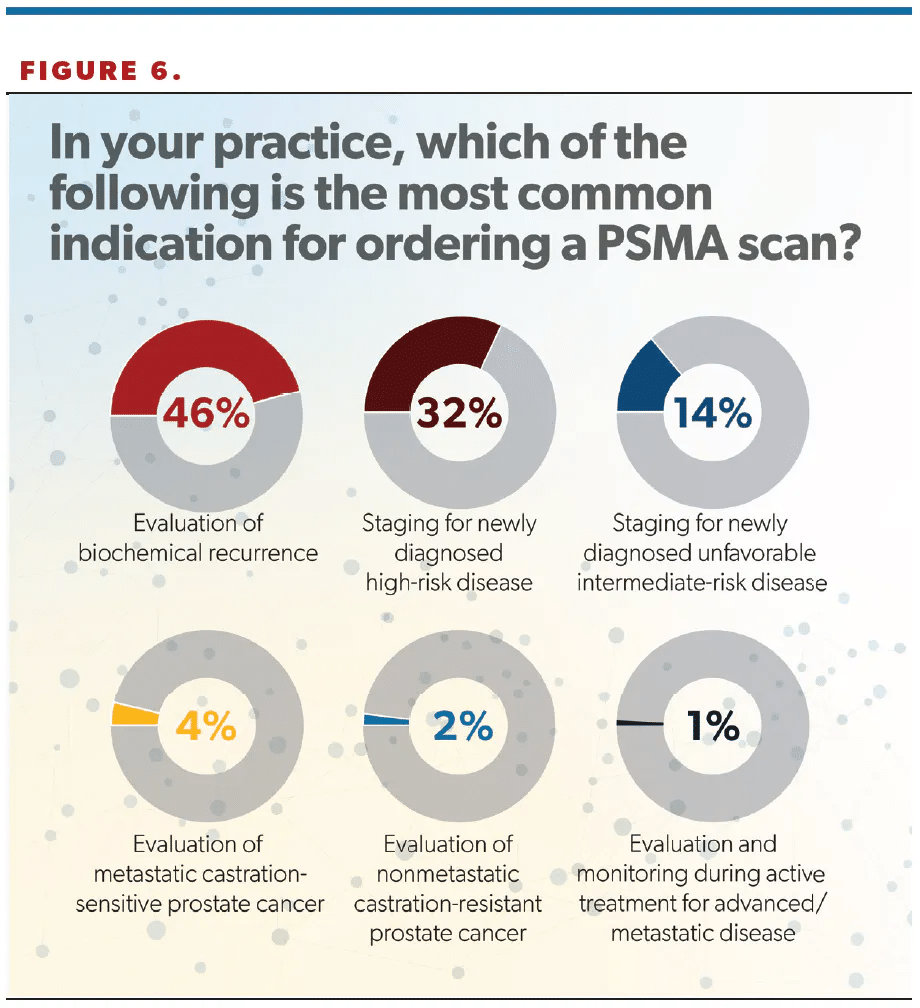
Nearly half (46%) of respondents gave “evaluation of biochemical recurrence” as the most common reason for ordering a PSMA scan; other indications included “staging for newly diagnosed high-risk disease” (32%) and “staging for newly diagnosed unfavorable intermediate-risk disease” (14%) (Figure 6).
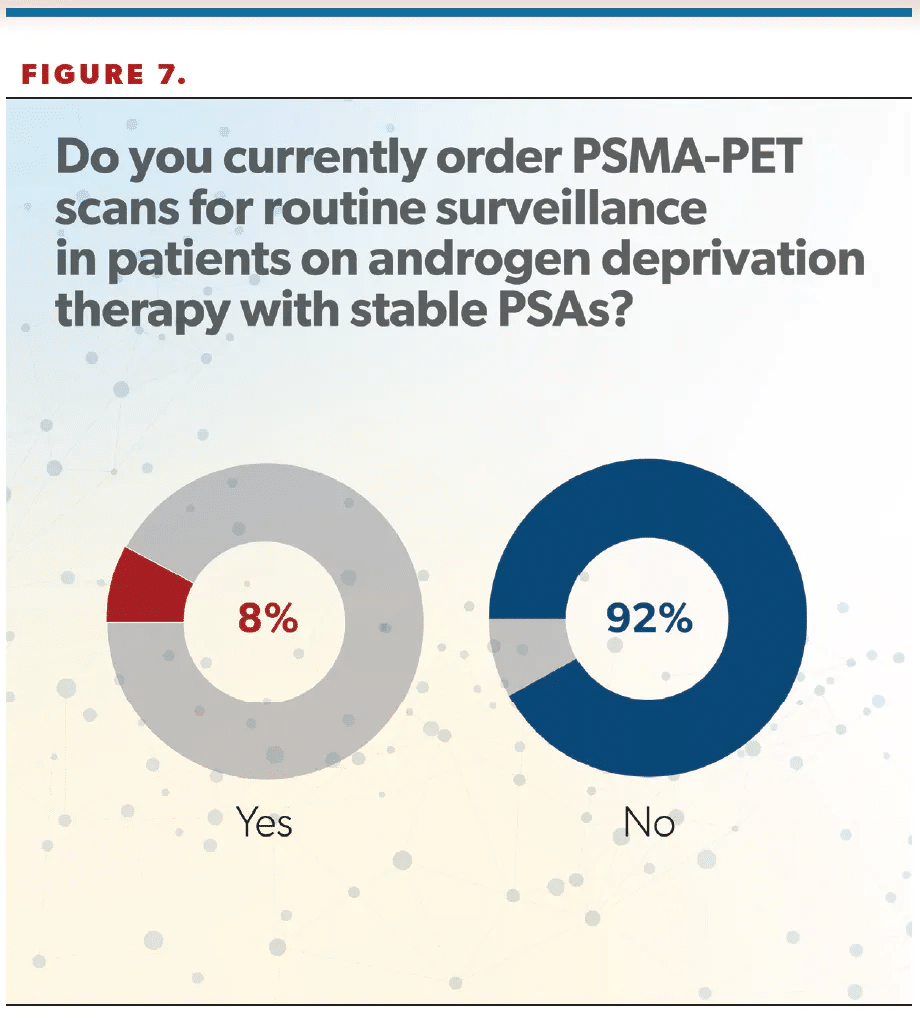
More than 90% of clinicians said they do not order PSMA-PET scans for routine surveillance in patients on androgen deprivation therapy with stable PSAs (Figure 7). Cookson said it was “surprising” to him that only 8% of urologists ordered scans in that scenario.
“I don’t know if that’s reality or if that was the way the question was worded, because maybe they weren’t thinking about a patient with metastatic disease. So it’s not fully vetted out how we’ll use these PSMA scans to monitor for progression. We tend to think if the PSA goes up, then something’s happening and that’s when we get the test. But there are reports of patients who progress even with relatively stable PSAs, and so how we interpose periodic surveillance imaging—whether that’s annually or at some defined interval—into the monitoring for men with metastatic disease on treatment remains to be defined. But it looks fairly underutilized based on the responses from this survey,” Cookson said.
Hafron also commented on this finding, saying, “I think that as we improve education and as urologists further understand how to use this awesome technology, I think we’ll see that number improve, and, ultimately, urologists will see the value of this technology.”
How the survey was conducted
This survey was developed by Urology Times Co–Editor in Chief Michael S. Cookson, MD, MMHC, FACS. It was created in Survey Monkey and fielded to the Urology Timesreadership via email and social media. The first question of the survey was designed to filter out responses from non–US-based practicing urologists. A total of 97 respondents completed the survey. Seventy-one percent of respondents had been in practice 21 years or longer. Fifty-four percent said they worked for an independent practice, and 74% said they were part of a practice of 3 or more physicians. Forty-four percent said they practiced in an urban, 40% in a suburban, and 16% in a rural/small town environment.

